We see many requirements for an electrically conductive bond formed with an adhesive. They vary from the micro (flip chips onto pcb’s, die-attach) to macro (wires onto sensors), and the range of projects is substantial. Often, our customers would like to use the adhesive in place of solder. The reasons include temperature restrictions, geometry, process considerations, or material compatibility.
We see many requirements for an electrically conductive bond formed with an adhesive. They vary from the micro (flip chips onto pcb’s, die-attach) to macro (wires onto sensors), and the range of projects is substantial. Often, our customers would like to use the adhesive in place of solder. The reasons include temperature restrictions, geometry, process considerations, or material compatibility.
In all these cases, we are joining two parts made of metal and/or with metal surface finishes (metallisation) – and therein lies the rub. Not all metallic surface finishes are suitable for electrically conductive adhesives, which are primarily formulated with silver (Ag) to achieve the conductivity (electrical, and sometimes thermal too).
Silver loaded conductive adhesives are often supplied pre-mixed and frozen
A potential failure mechanism is an increase in electrical resistance in the adhesive bondline after temperature and humidity aging, brought about by moisture and oxygen permeating the adhesive and interacting with the underlying metallisation, causing oxides to form. Additionally, the oxide bond is relatively weak to the substrate, and could result in an adhesive joint structurally failing at the oxide/metal interface.
The first thing to realise is that one of the reasons that silver is used as the filler in conductive adhesives is because silver oxides are electrically conductive. But that isn’t the case with other metals.
For high reliability conductive bonds, ideal surface finishes or metallisations include nobel metals which are resistant to oxidation in moist air – gold (Au), platinum (Pt), palladium (Pd) and silver (Ag). This includes the silver-palladium terminations on many surface mount components.
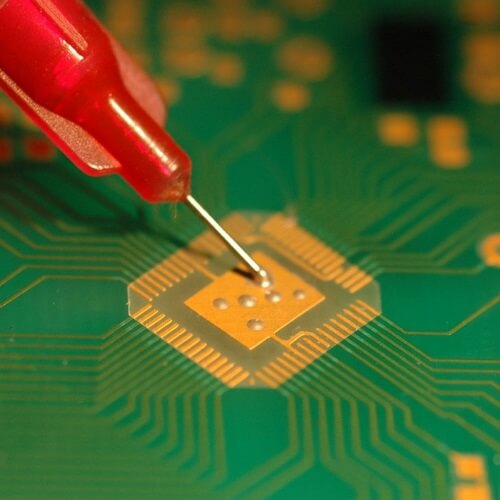
Good adhesive results can be obtained from nickel, brass and copper, although these will oxidise. Remove the oxides on these metals before bonding. Best practice would include adding a nickel/gold flash onto a copper surface prior to bonding.
The conductive bonding of tin/lead or pre-tinned surfaces, lead or aluminium should be only be undertaken with some caution. The oxides of these form readily and are non-conductive, leading to high and often unstable electrical contact resistance and reduction of shear strength. Also, the differing potentials of, say, Ag and Sn can lead to galvanic corrosion.
In evaluating your adhesive/substrate combination, we recommend that representative assemblies be tested under expected operating conditions, including temperature and humidity. Improvements in reliability might be achieved by the use of conformal coating or encapsulation.
Categories: adhere academy, electrically conductive, microelectronics